Heat Capacity of Calorimeter
T 2 T 1 is the temperature difference before and after heating or cooling K. Place one litre 1 kg of water in the calorimeter.

Specific Heat Capacity Physics Lessons Science Teaching Resources Science Facts
In the 18th and 19th centuries scientists abandoned the idea of a physical caloric and instead understood.

. It should now be becoming clear how convenient this specific heat capacity test is because the only thing to do once the experiment is on the. In the early modern period heat was thought to be a measurement of an invisible fluid known as the caloricBodies were capable of holding a certain amount of this fluid leading to the term heat capacity named and first investigated by Scottish chemist Joseph Black in the 1750s. The specific heat also called specific heat capacity is the measure of the heat energy that a substance in a unit quality absorbs or releases when the.
We will be measuring the change in temperature of the water in the calorimeter which lets us calculate the change in heat of the water in the calorimeter which we know to be equal and opposite to the change in heat of the sample. Q mc Delta T. Specific heat capacity is the most useful quantity available from DSC because it is directly related to sample properties and.
Heat loss by the fuel is equal to the heat gained by the water. 25772 JK-1 Mass of water and Calorimeter and stirrer. In case the sample occurs endothermic or exothermic phenomena such as transition and reaction this endothermic or exothermic phenomena is compensated by heat sink.
The only thing you need to remember is that you have to use consistent units for mass. Identify what is releasing heat and what is gaining heat for a given calorimetry experiment. To calculate the energy required to raise the temperature of any given substance heres what you require.
The below-mentioned formula can be used to calculate specific heat capacity values. Mass of the calorimeter and stirrer. It is usually expressed as calories per degree in terms of the actual amount of material being considered most commonly a mole the molecular weight in grams.
If you have it in Jkg C then you need the mass of the substance in kilograms. Heat sink has the enough heat capacity compared to the sample. Both solutions were originally at 261C.
The heat capacity in calories per gram is called specific heat. Clamp the thermometer into the smaller hole with the stirrer. The water increases in temperature by 10 degrees C.
A sealed immersion heater and a built-in heat exchanger both operated by the calorimeter controller provide precise jacket temperature control. In the previous article we discussed the specific heat capacity of substances. The definition of the calorie is based on the specific heat of water defined.
Such measurements can be made easily with this. This will require 2669 kJ of heat energy. The SI unit of specific.
Ii specific heat capacity C g of the solution. If initially the temperature of the water is 200C and after burning the nuts in the calorimeter we measure a water temperature of 333C then the change in temperature of the water T f - T i equals 133C and the heat captured by the calorimeter Q water is 150 g 0001 Calg C 133C or 20 Cal. Heat capacity of calorimeter C183JC Increase in temperature of calorimeter question_answer Q.
The mass of the material m The temperature change that occurs DeltaT The specific heat capacity of the material c which you can look up. Q is the heat absorbed or released by a material J. After the reaction the final temperature is 328C.
A Reliable Oxygen Bomb. We would like to show you a description here but the site wont allow us. Heat flow is proportional to the heat difference of heat sink and holders.
Total mass of the solution specific heat of the solution change in temperature of the solution. Therefore specific heat capacity c Qm Delta T. Soc 1929 51 2738.
If you have a specific heat capacity in Jg C then you need the mass of the substance in grams. A 435-g sample of copper at 999 C is dropped into a beaker containing 156 g of water at 187 C. 0068Kg Thermal Capacity of the Calorimeter and Stirrer.
Experiments were carried out using a MicroCal PEAQ-ITC calorimeter with MicroCal PEAQ-ITC Software version 13 both Malvern at 5 C in 20 mM Na 2 HPO 4 pH 75 150 mM NaCl or SPG buffer pH 50. The vessel is filled with water and the fuel is burned leading to the heating of the water. Thus the temperature difference between the sample and the.
Assuming that all the solutions have a density of 10 gcm3 and a specific heat capacity of 418 JCg calculate the enthalpy change for the neutralization of HCl by NaOH. Calculate the heat gained or released by a solution q solution involved in a given calorimetry experiment. All data Tsonopoulos and Ambrose 1995.
Specific Heat Capacity c Specific heat capacity of any substance is defined as the amount of heat required to change the temperature of a unit mass of the substance by 1 degree. Science Chemistry QA Library In a coffee-cup calorimeter 1200 mL of 10 M NaOH and 1200 mL of 10 M HCl are mixed. 0145Kg Mass of water 0077 Thermal capacity of water.
A calorimeter is an object used for calorimetry or the process of measuring the heat of chemical reactions or physical changes as well as heat capacityDifferential scanning calorimeters isothermal micro calorimeters titration calorimeters and accelerated rate calorimeters are among the most common types. Heat capacity ratio of heat absorbed by a material to the temperature change. This is the amount of heat required to raise 1 gram of that substance by 1C.
M is the mass of a material g. The entropy and the free energy of formation J. Q m c T.
Say in a calorimeter a fixed amount of fuel is burned. Place the immersion heater into the central hole at the top of the calorimeter. The heat capacity of toluene from 14 deg K to 298 deg K.
Assume that because all of the solutions are dilute aqueous solutions the specific heat capacity of each solution and hence the specific heat capacity of the final solution C g is the same as water which we will assume is 418 J g-1 C-1 C g 418 J g-1 C-1. The Parr 1108P Oxygen Bomb furnished with the calorimeter will safely burn samples liberating up to 8000 calories per charge using oxygen charging pressures up to 40 atm. An alternative truly measuring at operational conditions lies in using an adiabatic flow calorimeter and thus evaluating the enthalpy balance of a small quantity of the HTF in a bypass of the system 5.
C is the specific heat of a material JgK. Ive been tasked with working out the Latent heat of steam the following are all my results and values used. Unlike differential scanning calorimeters adiabatic flow.
A simple calorimeter just consists of a thermometer attached to a.

Constant Volume Calorimetry For More Precise Work Than The Coffee Cup Calorimeter The Heat Capacity Of The Entire C Coffee Cups Chemistry Education Chemistry
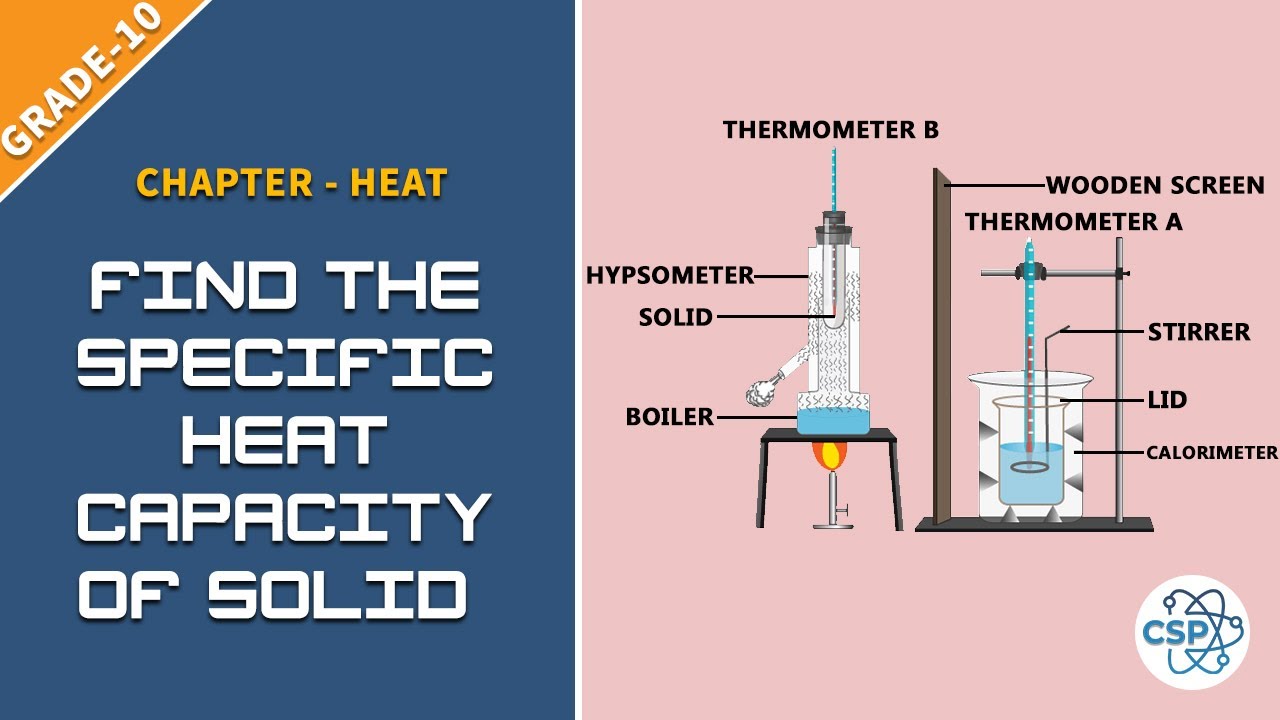
To Find The Specific Heat Capacity Of Solid By Using Method Of Mixtures See Class 10 Physical Properties Heat Science Experiments

50 Calorimetry Worksheet Answer Key Chessmuseum Template Library Worksheets Capacity Worksheets Letter Reversals

Pin By Redacted On Chemistry Education Chemistry Education What Is Science Ap Chem
No comments for "Heat Capacity of Calorimeter"
Post a Comment